Preclinical Trial Support
Toxicology Studies & Services
ATRPL Testing and Research offers an array of NABL and non-NABL Preclinical toxicology studies to its customers. The studies, in which our Toxicology services team has expertise, are composed of acute, sub-acute, and sub-chronic studies in various test systems with different routes of administration, along with in-vitro/in-vivo genotoxicity studies. These studies are performed in compliance with global regulatory guidelines, ISO, USP, IP, EP OECD, etc. at ATRPL in India.
We provide a comprehensive range of toxicology and safety pharmacology services by international regulatory guidelines. Phase, we assist in toxicological safety assessment to identify and eliminate the unsafe elements at the earliest stage in drug development with routine screening of compounds in the R&D.
ATRPL has the latest facilities to evaluate the different products in a professional manner that results in the timely delivery of the reports. The rich experience of our scientific team helps our customers meet their specific preclinical and other early-stage product development needs, enhance their decision-making process, and save their time and resources.
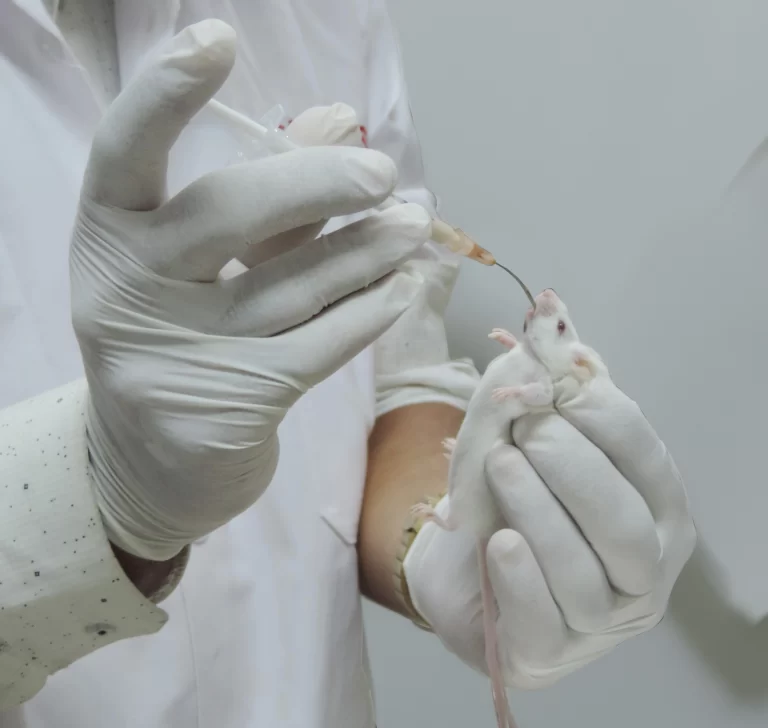
In Vivo Toxicology Services
Acute Studies
- Single-dose toxicology studies in rodents (Rats/Mice)
- Single-dose toxicology studies in non-rodents (Rabbits)
- Repeated Dose Toxicity Studies in Rodents (Rats/Mice/Guinea pigs) and Non-Rodents (rabbits)
- Sub-acute Toxicity: 14 days DRF and 28 days with toxicokinetic studies
- Sub-chronic Toxicity: 90 days with toxicokinetic studies
Different Routes of Administration
- Oral through gavage
- Dermal
- Subcutaneous
- Intraperitoneal
- Intramuscular
- Intravenous
- Pulmonary
- Trachel
In-vitro Toxicology Services
- Bacterial Reverse Mutation Test (Ames Test) using Salmonella Typhimurium
- In-vitro Mammalian Chromosome Aberration Test
- Cytotoxicity: Direct contact method and Extract Method
Pathology Services
- We offer Pathology Services (tissue processing & reporting) for toxicology and efficacy studies conducted
- Apart from above extensive list, we also take up research and development as part of any new protocol/study if needed by clients.
